Our group studies the biological principles and host interactions that underpin human virus replication and pathogenesis, seeking also to illuminate fundamental aspects of cell function and to pinpoint potential strategies for viral control or eradication. One fruitful approach that we have employed is the analysis of “context-dependent” deficiencies in virus replication.
These may be manifested during the examination of viral mutants/variants, cell-type or species-specific effects, or altered cell culture conditions, and, simplistically, may be attributed either to 1) the lack of cellular dependency factors that promote replication or 2) the presence of natural suppressors (frequently called restriction factors).
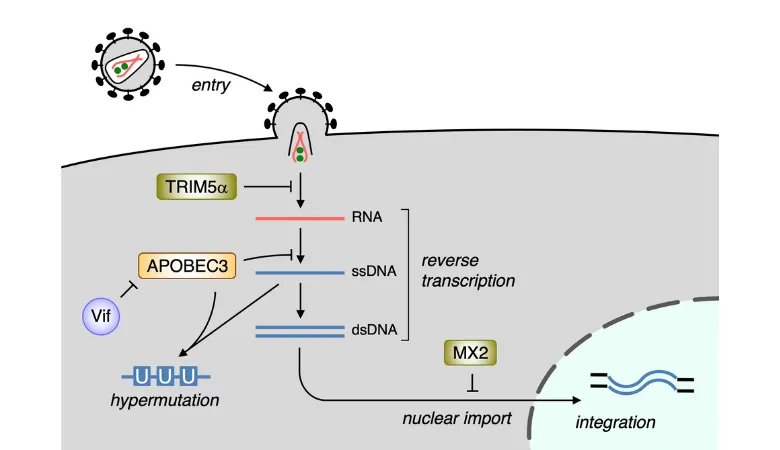
HIV-1 Post-Entry Restrictions. TRIM5alpha and MX2 reside in target cells and block early reverse transcription and viral nuclear import, respectively. APOBEC3 proteins are incorporated into virus particles and interfere with viral replication complexes by editing cDNA via cytidine deamination and suppressing reverse transcription. HIV-1 encodes an antagonist for APOBEC3 proteins, called Vif, but not for TRIM5alpha or MX2.
Current PhD students:
