Cardiovascular Biobank
This page provides an overview of the Cardiovascular Biobank, a crucial resource for advancing translational research in cardiovascular and metabolic medicine. Established in collaboration with NHS Trusts and King’s College London, the biobank aims to enhance the understanding of cardiovascular conditions through the collection of tissue samples and comprehensive clinical data. This initiative fosters collaboration among clinical and non-clinical academics, promoting significant advancements in research and patient care.
About Us
The Cardiovascular Biobank was established in 2018 and is currently situated within the King’s Academic Department of Vascular Surgery, South Bank Section, St Thomas’ Campus. In 2024, we received funding from the KHP Centre for Translational Medicine, which has enabled a significant expansion of our resources.
We have obtained ethical approval to collect a variety of biological samples, including blood, urine, DNA, and tissue samples, along with clinical data from consenting patients receiving care at King’s Health Partners (KHP) organisations. These organisations include Guy’s and St Thomas’, Royal Brompton and Harefield, and King’s College Hospital.
Our Mission
The Cardiovascular Biobank serves as a pivotal initiative in driving translational research by:
- Facilitating Access: Providing clinicians, academics, and external collaborators with access to valuable tissue samples.
- Enhancing Understanding: Aiming to deepen the understanding of cardiovascular conditions through the integration of biological findings with comprehensive clinical data.
- Promoting Collaboration: Encouraging collaboration between clinical and non-clinical academics, as well as fostering cross-site cooperation among scientists and interventionalists.
Information for Patients
If you are a patient interested in learning more about the sample biobanking process, please visit our dedicated Information for Patients page. Here, you will find detailed information about how samples are collected, the consent process, and the potential benefits of participating in the biobank.
By participating in the Cardiovascular Biobank, you contribute to a vital resource that supports ground breaking research aimed at improving cardiovascular health and patient outcomes.
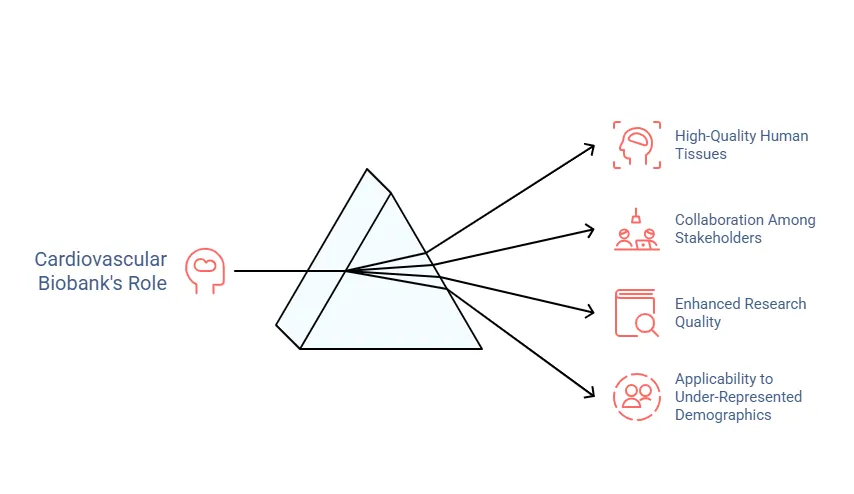
Vision and aims
One of the most significant obstacles faced by researchers is a lack of access to high-quality human tissues that have been collected and stored in an appropriate, standardised manner. The associated clinical data is often sparse and recorded only at the time of sampling.
The Cardiovascular Biobank provides a platform for testing discoveries using tissues from our ethnically diverse patients, increasing the likelihood of broader applicability, particularly among under-represented demographics. Additionally, the collaborative efforts between researchers, clinicians and industry partners enable swift testing and implementation of initiatives on a large scale, thanks to KHP’s extensive clinical network.
This strategic approach holds great promise for driving meaningful progress in cardiovascular health. Our aim reflects a comprehensive approach to advancing understanding and treatment options for cardiovascular disorders, emphasising both the quality of research data and the power of collaboration.
Sample collection overview
We are building a collection of frozen samples from cardiovascular disease areas and healthy volunteers. Samples are collected from patients with:
- Aortic aneurysms
- Intermittent claudication
- Inherited cardiac conditions/heart failure
In addition to the biological samples, we meticulously record accompanying clinical information in a secure repository sourced from electronic health records. This data encompasses a wide range of information, including, but are not limited to:
- Patient demographics
- Medical history and co-morbidities
- Treatment history
- Medications
- Routine lab results
Requesting New Sample Collections
If the specific samples you require are not currently being collected, we invite you to submit a request for us to initiate a new prospective collection. This can be tailored for:
- A defined research project
- Establishing a new cohort that may provide valuable insights in the future
- Providing sample types as fresh tissue.
To ensure that all requests are handled appropriately, we have established a dedicated committee to review each request on a case-by-case basis.
Hosting Samples from Other Collections
In addition to our own sample collection efforts, we are also equipped to host samples from other collections. This initiative aims to broaden the scope of available samples and enhance collaborative research opportunities. For further inquiries or to submit a request, please contact our team. We look forward to supporting your endeavours in the field of cardiovascular research.

KHP Cardiovascular Biobank Newsletter Issue 1, March 2025
The Cardiovascular Biobank is generously supported by the Centre for Translational Medicine with the aim of building a comprehensive collection of human samples and associated clinical data from patients treated for cardiovascular conditions across King’s Health Partners (KHP) clinical sites. In the inaugural edition of the newsletter we will introduce the team and offer an overview of the biobank, including where to access additional information. We are grateful for the ongoing support from partners across KHP in helping to develop this valuable resource.
Researchers are expected to acknowledge the Cardiovascular Biobank in any publications which utilise data and/or samples from the Biobank.
Licences and Ethics
The Cardiovascular Biobank has been reviewed and given an ethically favourable opinion by the NRES London-Fulham Committee and operates under approval 23/LO/0506 and HTA 12522 and 12293.
How we are governed
The Cardiovascular Biobank is governed by its Terms of Reference, which include the following key committees:
1. Management Committee
The Management Committee has overall responsibility for access, operations, compliance, audit, and governance of the research biobank. This committee meets at 6-monthly intervals and includes members such as:
- The chair
- A representative from GSTT, RBHH, and KCH
- A representative from KCL
- An HTA-designated person
- The biobank manager
- An independent member for the Committee.
2. Operational Group
The Operational Group advises on Biobank operation matters and monitors compliance with HTA license requirements, standardisation of laboratory procedures, and quality assurance. This group meets every 3 months and includes:
- The chair
- Clinical lead
- The biobank manager
- A data controller.
3. Access Committee
The Access Committee is responsible for reviewing applications to access tissue and/or clinical data for research purposes. It meets every 3 months, but ad hoc meetings for urgent sample requests may be arranged. Members of the Access Committee include:
- The chair
- Consultant Clinician Representatives from KCL, GSTT, KCH or RBHH or Independent Scientist
- The Biobank manager
- An external expert representative
- An HTA Designated person.
Accessing the Biobank
We welcome applications for sample access from NHS organisations, academic institutions and commercial organisations involved in research. It is strongly recommended that you contact the biobank at the earliest possible opportunity if you plan to utilise samples for an upcoming project. For preliminary discussions regarding your project, please reach out to us at cardiovascular.biobank@kcl.ac.uk.
You can apply to use samples already stored in the biobank, or make a request to set up a new sample collection. If you wish to make an application please complete the sample access form and return it to us via email.
Applications will be reviewed by the Management Access Committee, which will evaluate requests base on:
- Scientific merit
- Study design
- Requestor’s financial resources (i.e. details of grant to fund study)
- The relevant registry resources
Each application will be reviewed by at least three members of the access committee, except where the accelerated procedure applies. The review of applications will be completed within two weeks of receipt from the manager or coordinator and the summarising of review comments and reporting will be undertaken by the clinical lead for the biobank within two weeks of receipt of the review forms.
A Material Transfer Agreement (MTA) must be in place before the project can begin.
Publications
Researchers are expected to acknowledge the Cardiovascular Biobank in any publications which utilise data and/or samples from the Biobank.
Vision and aims
One of the most significant obstacles faced by researchers is a lack of access to high-quality human tissues that have been collected and stored in an appropriate, standardised manner. The associated clinical data is often sparse and recorded only at the time of sampling.
The Cardiovascular Biobank provides a platform for testing discoveries using tissues from our ethnically diverse patients, increasing the likelihood of broader applicability, particularly among under-represented demographics. Additionally, the collaborative efforts between researchers, clinicians and industry partners enable swift testing and implementation of initiatives on a large scale, thanks to KHP’s extensive clinical network.
This strategic approach holds great promise for driving meaningful progress in cardiovascular health. Our aim reflects a comprehensive approach to advancing understanding and treatment options for cardiovascular disorders, emphasising both the quality of research data and the power of collaboration.

Sample collection overview
We are building a collection of frozen samples from cardiovascular disease areas and healthy volunteers. Samples are collected from patients with:
- Aortic aneurysms
- Intermittent claudication
- Inherited cardiac conditions/heart failure
In addition to the biological samples, we meticulously record accompanying clinical information in a secure repository sourced from electronic health records. This data encompasses a wide range of information, including, but are not limited to:
- Patient demographics
- Medical history and co-morbidities
- Treatment history
- Medications
- Routine lab results
Requesting New Sample Collections
If the specific samples you require are not currently being collected, we invite you to submit a request for us to initiate a new prospective collection. This can be tailored for:
- A defined research project
- Establishing a new cohort that may provide valuable insights in the future
- Providing sample types as fresh tissue.
To ensure that all requests are handled appropriately, we have established a dedicated committee to review each request on a case-by-case basis.
Hosting Samples from Other Collections
In addition to our own sample collection efforts, we are also equipped to host samples from other collections. This initiative aims to broaden the scope of available samples and enhance collaborative research opportunities. For further inquiries or to submit a request, please contact our team. We look forward to supporting your endeavours in the field of cardiovascular research.
Facility staff

KHP Cardiovascular Biobank Newsletter Issue 1, March 2025
The Cardiovascular Biobank is generously supported by the Centre for Translational Medicine with the aim of building a comprehensive collection of human samples and associated clinical data from patients treated for cardiovascular conditions across King’s Health Partners (KHP) clinical sites. In the inaugural edition of the newsletter we will introduce the team and offer an overview of the biobank, including where to access additional information. We are grateful for the ongoing support from partners across KHP in helping to develop this valuable resource.
Researchers are expected to acknowledge the Cardiovascular Biobank in any publications which utilise data and/or samples from the Biobank.
Licences and Ethics
The Cardiovascular Biobank has been reviewed and given an ethically favourable opinion by the NRES London-Fulham Committee and operates under approval 23/LO/0506 and HTA 12522 and 12293.
How we are governed
The Cardiovascular Biobank is governed by its Terms of Reference, which include the following key committees:
1. Management Committee
The Management Committee has overall responsibility for access, operations, compliance, audit, and governance of the research biobank. This committee meets at 6-monthly intervals and includes members such as:
- The chair
- A representative from GSTT, RBHH, and KCH
- A representative from KCL
- An HTA-designated person
- The biobank manager
- An independent member for the Committee.
2. Operational Group
The Operational Group advises on Biobank operation matters and monitors compliance with HTA license requirements, standardisation of laboratory procedures, and quality assurance. This group meets every 3 months and includes:
- The chair
- Clinical lead
- The biobank manager
- A data controller.
3. Access Committee
The Access Committee is responsible for reviewing applications to access tissue and/or clinical data for research purposes. It meets every 3 months, but ad hoc meetings for urgent sample requests may be arranged. Members of the Access Committee include:
- The chair
- Consultant Clinician Representatives from KCL, GSTT, KCH or RBHH or Independent Scientist
- The Biobank manager
- An external expert representative
- An HTA Designated person.
Accessing the Biobank
We welcome applications for sample access from NHS organisations, academic institutions and commercial organisations involved in research. It is strongly recommended that you contact the biobank at the earliest possible opportunity if you plan to utilise samples for an upcoming project. For preliminary discussions regarding your project, please reach out to us at cardiovascular.biobank@kcl.ac.uk.
You can apply to use samples already stored in the biobank, or make a request to set up a new sample collection. If you wish to make an application please complete the sample access form and return it to us via email.
Applications will be reviewed by the Management Access Committee, which will evaluate requests base on:
- Scientific merit
- Study design
- Requestor’s financial resources (i.e. details of grant to fund study)
- The relevant registry resources
Each application will be reviewed by at least three members of the access committee, except where the accelerated procedure applies. The review of applications will be completed within two weeks of receipt from the manager or coordinator and the summarising of review comments and reporting will be undertaken by the clinical lead for the biobank within two weeks of receipt of the review forms.
A Material Transfer Agreement (MTA) must be in place before the project can begin.
Publications
Researchers are expected to acknowledge the Cardiovascular Biobank in any publications which utilise data and/or samples from the Biobank.
Contact us
Please contact the biobank team for more information. Cardiovascular Biobank King’s Academic Section of Vascular Surgery 1st Floor North Wing St Thomas’ Hospital London SE1 7EH




