Vitreous Thin Film
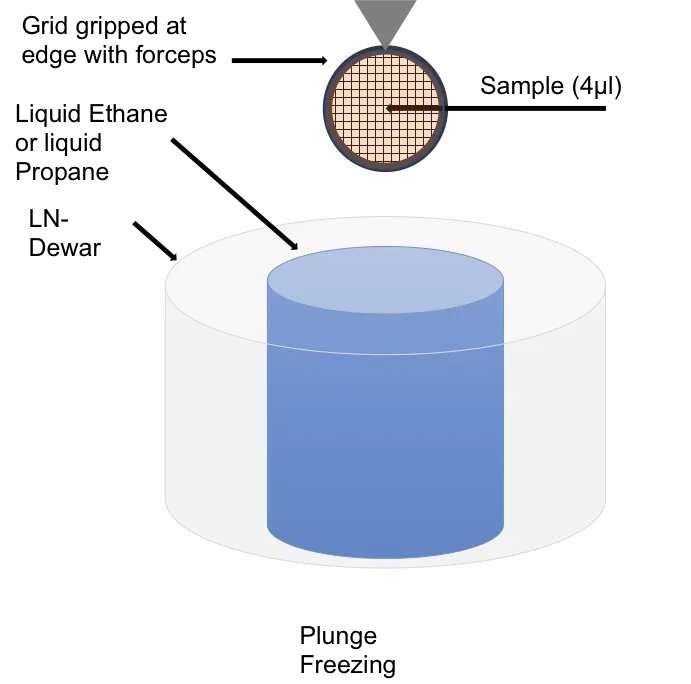
Plunge freezing methods for vitrifying and creating a vitreous thin film (VTF) sample for cryogenic electron microscopy have been widely adopted.
Cryo-immobilisation vitrifies the sample in milliseconds, locking all the macromolecular components, elements and structures as they were in the living state for very high resolution analysis.
The ultimate goal is to derive the structures of molecular machineries of living systems within their natural, functional contexts and their interactions with other observed components.
Vitreous thin film advantages:
- fast immobilisation with no loss of time resolution
- preservation of structure
- rapid simple technique for small particles
- no loss of ions/molecules or lipids
- no depolymerization of proteins
- no denaturation of enzymes
- no osmotic effects.
Examples of use
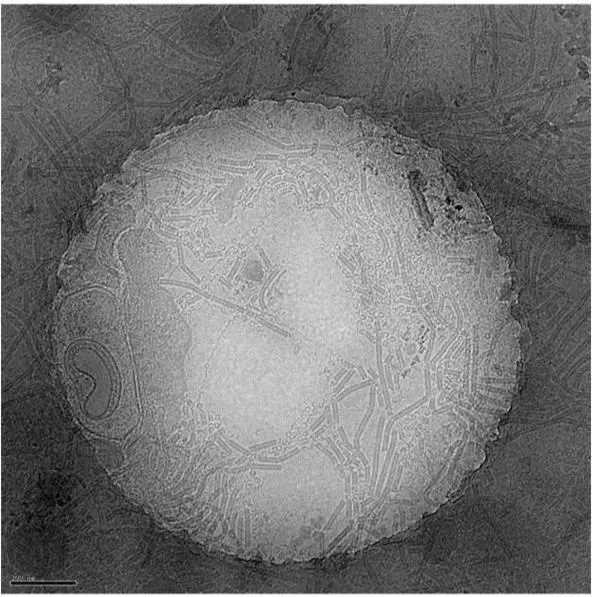
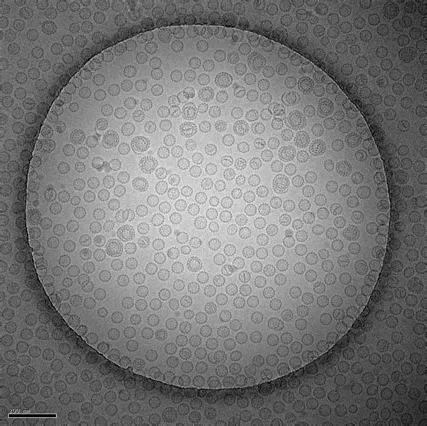
Plunge freezing is routinely used to prepare small sample volumes mounted on transmission electron microscopy (TEM) grids for cryo TEM.
Plunge freezing is suitable for the study of isolated proteins and macromolecular complexes with the final aim of resolving their 3D structure.
Plunging cells grown directly on grids can be used to prepare material for cryogenic field emission gun scanning electron microscopy, where cell surfaces are of interest, as well as for investigating cellular processes that occur at the cell periphery (eg endocytosis) using TEM.
Equipment available
- Leica-microsystems EM GP.