Cryogenic Electron Microscopy Of Vitreous Sections
Cryogenic transmission electron microscopy (cryo TEM) of thin vitrified films of aqueous suspensions allows small biological particles to be observed in their native state (without artifacts from dehydration and staining). However, larger samples (such as cell monolayers and tissues) cannot be squeezed to a thin enough film for cryo TEM. A different approach to preparing a thin section is required. Cryo-ultramicrotomy provides a solution.
Successful cryogenic electron microscopy of vitreous sections (CEMOVIS) requires vitrification, normally by high pressure freezing, of a piece of biological material which is sectioned using a cryo-ultramicrotome. These thin sections (~50nm) are then observed in the vitrified state using a cryogenic transmission electron microscope.
In general, samples seen with CEMOVIS are very different from what is seen in resin-infiltrated tissue as membranes are seen in more detail and are closer to their native state. CEMOVIS has the highest potential when combined with electron tomography for 3D reconstruction.
CEMOVIS has not been routinely adopted, principally because of the technical difficulty of producing ribbons of frozen sections on a transmission electron microscopy grid. A double micromanipulator tool that guides the ribbon using an electrically conductive fibre, and then positions and attaches the ribbon to a TEM grid, is available in the centre to simplify the technique.
Examples of use
CEMOVIS is the ideal cryo-ultramicrotomy technique to support the study of cells and tissues in their native state by cryo TEM or cryo TEM tomography.
The routine workflow for this technique involves:
- high pressure freezing the specimen
- cryo-ultramicrotomy assisted by micromanipulators
- viewing by cryo TEM microscope
Equipment
- JEOL JEM-F200
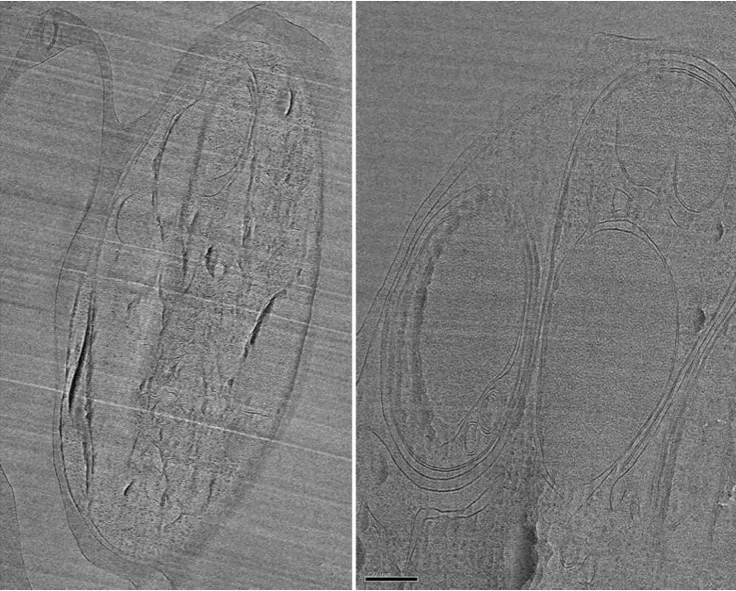
CEMOVIS reveals exceptional details of membrane structure and organisation. However, samples are subject to compression and appear squashed in the direction of cutting. Small scratches in the section are normal. (In collaboration with Saibil, Birkbeck and Blackman, Crick, MacLellan-Gibson, NIBSC).
