Electron cryo-tomography, instead, allows us to obtain detailed and artefact-free 3D images of the frozen muscle.
Professor Stefan Raunser, Max Planck Institute of Molecular Physiology
24 March 2021
Researchers use cryo-electron tomography to reveal novel molecular details of muscle sarcomeres
A group of researchers succeed in producing the first ever high resolution image of the sarcomere.
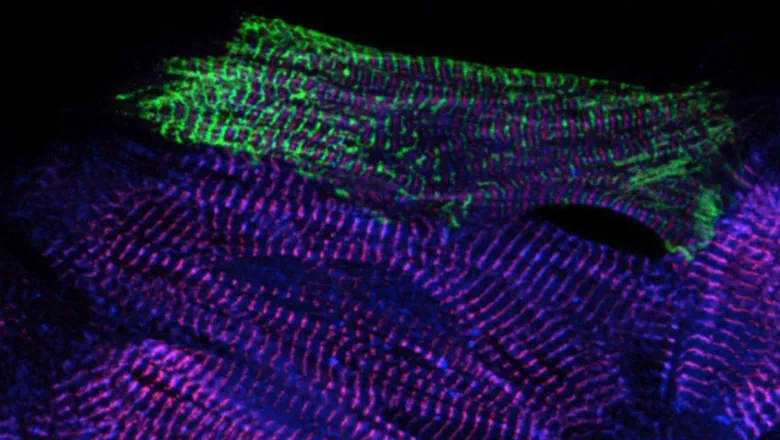
A team of international researchers, led at King's by Professor Mathias Gautel from the School of Basic and Biomedical Sciences, have produced the first high-resolution 3D image of the sarcomere, which is the basic contractile unit of skeletal and heart muscle cells.
This work, a collaboration with Professor Stefan Raunser, Director at the Max Planck Institute of Molecular Physiology in Dortmund, uses cryo- electron tomography, which is able to reveal an image of the structures directly in frozen muscle cells, an insight which could translate into future medical treatments for muscle diseases.
Sarcomeres are small repeating subunits of myofibrils, the long cylinders that bundle together to form muscle fibres. So far, traditional experimental approaches to investigate the structure and function of muscle tissue were performed on reconstructed protein complexes or suffered from low resolution.
Researchers flash-freeze the biological sample at a very low temperature (- 175 °C). Through this process, the sample preserves its hydration and fine structure and remains close to its native state. FIB milling is then applied to shave away extra material and obtain an ideal thickness of around 100 nanometres for the transmission electron microscope, which acquires multiple images as the sample is tilted along an axis. Finally, computational methods reconstruct a three-dimensional picture at high resolution.
Our King’s team have worked with the Dortmund team for over four years, in order to optimise the preparation of mouse myofibrils suitable for cryo-ET applications. This eventually resulted in a resolution of one nanometre.
It is so exciting to see sarcomeres with details thought unimaginable only four years ago, in the absence of any artefacts caused by chemical fixation, or the heavy-metal staining used traditionally in electron microscopy.
Professor Mathias Gautel, School of Basic & Medical Biosciences
The scientists used a sample with myosin strongly bound to actin, representing a stage of the contracting muscle that is called the rigor state. For the very first time they were able to visualise how two heads of the same myosin bind to an actin filament in the native cell. They also discovered that the double head not only interacts with the same actin filament but is also found split between two actin filaments.
This has never been seen before and shows that proximity to the next actin filament is stronger than the cooperative effect between the neighbouring heads.
This is just the beginning. Cryo-ET is moving from niche to widespread technology in structural biology. Soon, we will be able to investigate ageing and muscle diseases at the molecular and even atomic level, using mouse and zebrafish models generated at King’s.
Professor Mathias Gautel, School of Basic & Medical Biosciences

