15 December 2017
Nanomaterial may enable new chemical processes
Reactive Plasmonics researchers at King’s College London have engineered a new nanoscale device which creates a controlled stream of ‘hot electrons’ – high energy electrons which allow unusual chemical reactions to take place.
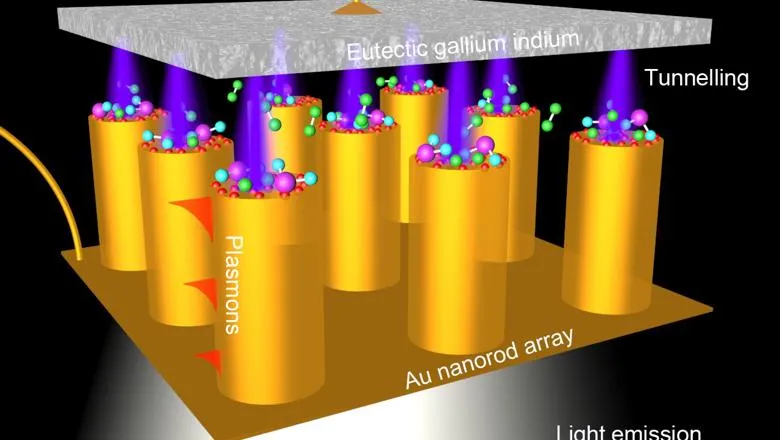
The research, published in Nature Nanotechnology, describes how a metamaterial – a material with properties not found in nature – can be constructed and use quantum effects to turn electrons flowing through a circuit into hot electrons and light, in a highly controlled manner.
Applying a voltage across the metamaterial causes a flow of electrons from one material to another. The materials are separated by an air gap, which would usually stop the electron flow. However, as the air gap is less than a nanometer the electrons can ‘tunnel’ through – known as electron tunnelling – thanks to quantum mechanics. Most of the tunnelling electrons arrive as ‘hot electrons’ in the device’s gold nanorod tips. They are of great interest to chemical industries, since their high energies allow chemical reactions to occur between molecules which would not normally react with each other. A small proportion of the tunnelling electrons excite other particles (called plasmons) in the metamaterial causing light to be emitted. Usually this conversion of energy is very inefficient but the huge number of tunnel junctions improves the rate of conversion, making the emitted light visible to the naked eye.
The creation of hot electrons is useful to a wide range of industries that are interested in making new chemicals that do not occur under normal conditions. For example, synthesising new molecules in pharma and chemical industries. The metamaterial can be used for developing and understanding new chemical reactions where precise stimulation and monitoring are paramount.
The material can reliably detect hydrogen and oxygen, two chemicals where there is a great need for sensitive monitoring. Detecting hydrogen leaks is important in the production of fuel cells, and monitoring the presence of oxygen is vital in a wide variety of controlled chemical reactions, such as in the manufacture of drugs. The material can be modified to be sensitive to different molecules, making it viable as a highly sensitive, cheap, and easy to use sensor to detect molecules.
The material could also be used in small scale electronics instead of bulky semiconductor lasers. Since it can generate light it can be used to transmit information between or within microchips by turning the light on and off to produce 1s and 0s.
Dr Pan Wang, lead author of the paper, says: “This one tiny device offers several amazing applications: plasmon excitation, light generation, and chemical reaction activation. And all this is achieved by a small, easy to produce material which only requires a small voltage to function.”
Principal Investigator Professor Anatoly Zayats concludes: “When we began these studies we expected to generate some weak light which we thought should be enough for various nanophotonic applications on a chip. But as sometimes happens in the research, the applications are much richer. We believe the potential of the approach for designing chemical reactions stimulated with hot electrons and monitoring chemical processes for drug and materials discovery is huge.”

