We next want to explore these systems in more complex environments which better reflect their cellular situation, especially in the context of the membrane lipid milieu which surround these proteins. Achieving improved structural insight into proteins within these contexts would be a significant step forward in understanding how they shape cellular function when in diseased/healthy states.
Dr Eamonn Reading
10 November 2020
Latest research from King's Chemists furthers understanding of multidrug resistance
Latest research from King’s chemists explores the role that protein dynamics play in the function and inhibition of multidrug-resistant activity in membrane proteins.
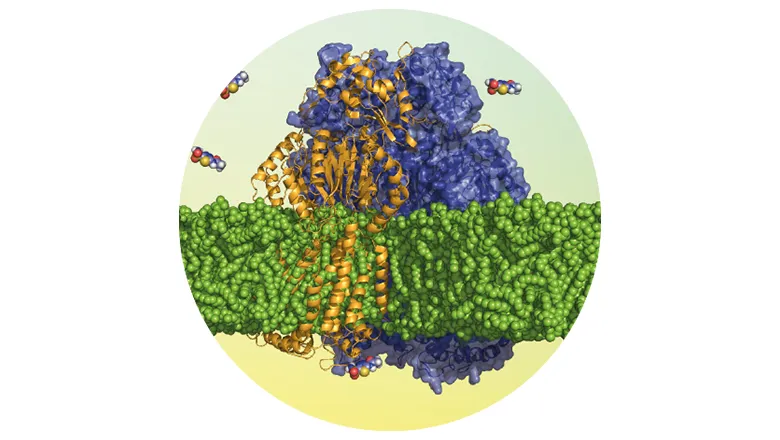
Research led by scientists at King’s, and published in Nature Communications, has produced a deeper understanding of the structural dynamics of membrane proteins, and its impact upon antibiotic and multidrug resistance (MDR). With a specific focus on multidrug efflux pumps – sets of proteins which recognise and pump out antimicrobials – the team’s research has revealed that an efflux pump inhibitor enhances biotic activity by restraining protein dynamics, rather than preventing antibiotic binding.
Currently, no clinically-approved pump inhibitors have been developed – partially due to insufficient understanding of protein mechanisms of action. Membrane proteins are key targets for more than half of modern drugs, and the team’s findings serve to further advance the battle against multidrug resistance, which has become a global societal challenge.
Dr Eamonn Reading, UKRI Future Leaders Fellow at King’s Department of Chemistry, elaborated further on the team’s next steps:
Using a combination of biochemical, computational, and structural mass spectrometry expertise, this collaborative research effort was conducted between the Reading, Booth and Politis groups at King’s Department of Chemistry, Professor Laura Piddock (University of Birmingham) and Dr Attilio Vargiu (University of Cagliari, Italy).
Commenting on the collaborative nature of the research, Eamonn said:
What was really exciting was working with expert microbiologists and computational chemists to guide and understand the data we obtain from our advanced mass spectrometry techniques – enabling the appreciation of how fundamental protein dynamics govern the drug efflux, inhibition, and acquired bacterial resistance of these important class of membrane proteins.
Dr Eamonn Reading

