The heart is the vital pump that keeps us alive by supplying nutrients and oxygen to all body organs. Defects in its structure and function lead to serious heart diseases, for many of which there are yet no effective cures. As we cannot repair what we don’t understand in detail, resolving the molecular architecture of the contractile machinery in its natural context is an essential step to understanding the molecular mechanism of normal contractions and the defects leading to heart disease. This study has revealed the unexpected complexity of the shapes of the molecules building the thick filaments that will be important to understand both normal and abnormal hearts. Our combined use of cutting-edge techniques has created exactly such a comprehensive picture.
Professor Mathias Gautel, British Heart Foundation Chair of Molecular Cardiology, King's College London
01 November 2023
Breakthrough discovery sheds light on heart and muscle health
An international team, led by the Max Planck Institute in Dortmund in collaboration with King's College London, have shot the first true-to-life 3D image of the thick filament of mammalian heart muscle
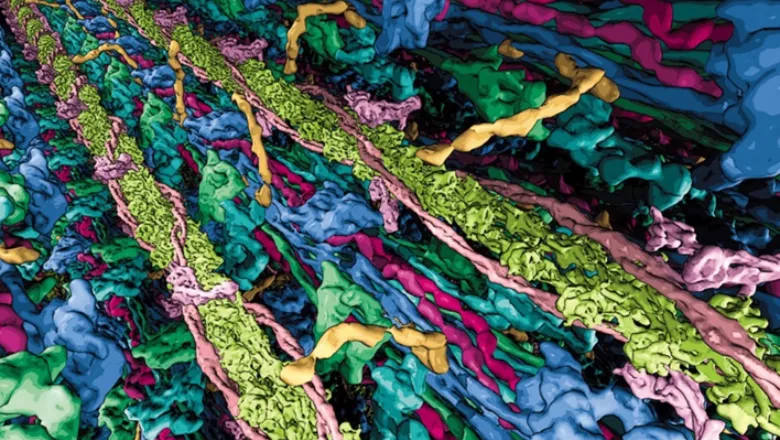
The human heart, often described as the body's engine, is a remarkable organ that tirelessly beats to keep us alive. At the core of this vital organ, intricate processes occur when it contracts, where thick and thin protein-filaments interact within the sarcomere, the fundamental building block of both skeletal and heart muscle cells. Any alterations in thick filament proteins can have severe consequences for our health, leading to conditions such as hypertrophic cardiomyopathy and various other heart and muscle diseases. Amazingly, understanding the molecular impact of such diseases-causing changes has so far been hindered by the lack of highly resolved molecular information of the filaments.
An international team, led by Stefan Raunser, Director at the Max Planck Institute of Molecular Physiology in Dortmund, in collaboration with Mathias Gautel, British Heart Foundation Chair of Molecular Cardiology at King's College London, have successfully obtained the first high-resolution 3D image of the heart thick filament in its natural cellular environment, utilizing cutting-edge techniques known as electron cryo-tomography and super-resolution fluorescence microscopy. These findings have recently been published in Nature.
Accomplishing this imaging offers a glimpse into the molecular organization and arrangement of the components within the thick filament. This newfound insight is nothing short of a crucial framework for comprehending how muscles operate in both health and disease. By understanding the intricate mechanics at play, scientists are now better equipped to develop innovative pharmacological approaches and treatments that can target heart and muscle disorders, potentially revolutionizing medical intervention in these areas.
The Max Planck Institute developed an electron cryo-tomography workflow specifically tailored to the investigation of muscle samples. This involved flash-freezing mammalian heart muscle samples, produced by the King’s team, at a very low temperature (-175 °C) and applying a focused ion beam (FIB milling) to thin out the samples to an ideal thickness for the transmission electron microscope. This acquires multiple images as the sample is tilted along an axis, a three-dimensional picture at high resolution is then reconstructed. To validate the molecular interpretation of the 3D images, the King’s team used super-resolution fluorescence microscopy that allows revealing the position of molecules with great precision.
If you want to fully understand how the muscle works on the molecular level, you need to picture its components in their natural environment - one of the biggest challenges in biological research nowadays that cannot be tackled by traditional experimental approaches.
Professor Stefan Raunser, Director, Structural Biochemistry, Max Planck Institute for molecular Physiology Dortmund
These methods have allowed the teams to produce the first high-resolution image of the cardiac thick filament spanning the sarcomere, giving insight into the thick filament’s molecular organization and its surprisingly complex function. Further investigation is now needed as the team predict that this allows the thick filament to sense and process numerous muscle-regulating signals and thus to regulate the strength of muscle contraction depending on the sarcomere region.
The next steps will include analysis of samples from animal models and patients with muscle disease, which will contribute to a better understanding of diseases like hypertrophic cardiomyopathy and to the development of innovative therapies.
Read the full paper: Structure of the native myosin filament in the relaxed cardiac sarcomere.

