Enzymes in nature carry out incredible transformations quickly and efficiently, in a way that is very difficult to reproduce in the lab. Our new model brings us one step closer to understanding how these biological systems do it so well, so we can design catalysts at an industrial scale to replicate the transformative ability of nature, to tackle key societal issues such as climate change."
Dr Rebecca Musgrave
18 July 2024
King's scientists make breakthrough replicating enzyme that captures carbon
The new model offers new understanding of how nature captures CO2 from the atmosphere.
Scientists from King’s College London have recreated the active site of Acetyl-CoA Synthase, an enzyme involved in capturing carbon from the atmosphere. The research, carried out in collaboration with Imperial College London, advances our understanding of this important enzyme, and offers a potential new solution to capture CO2 from the atmosphere in the fight against climate change.
Led by Dr Rebecca Musgrave from the Department of Chemistry and Dr Daniel Wilson from UCL, the team successfully recreated an active site – the site where the chemical reactions occur - of the enzyme Acetyl-CoA synthase (ACS). ACS transforms CO2 into ‘acetyl coenzyme-A’ – an essential molecule used in living beings. Their findings are published today in the Journal of the American Chemical Society.
ACS is most famously known for its role in the acetic acid cycle or Krebs Cycle - a series of chemical reactions in living things, in which acetic acid is oxidized to produce energy. It is therefore vital for storing and releasing energy and for capturing CO2 from the atmosphere and storing it as carbon. The team’s new model was able to replicate this chemical reaction in the lab, capturing atmospheric carbon and storing it as acetyl coenzyme-A.
Enzymes are proteins that function as biological catalysts by accelerating chemical reactions. As such, they carry out vital functions in nature, including in human biology. The chemical pathways created by enzymes have developed over billions of years into large, complex biological systems, and are therefore very challenging to study and replicate in the lab Scientists often recreate smaller, molecular versions of enzymes - models of the ‘active site’ in the lab, to study them.
The ACS enzyme is found in bacteria and some single-celled organisms and functions without oxygen, building complex organic molecules from carbon dioxide and hydrogen. Whilst attempts had been made to model the enzyme’s active site in the lab, they were not able to accurately replicate the shape and electronic environment of the active site to capture carbon.
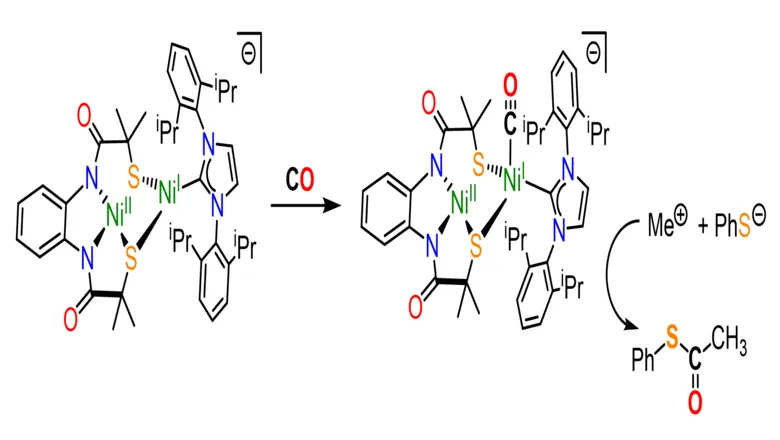
Dr Daniel Wilson, lead researcher from UCL, said: “Scientists have been studying the ACS enzyme for decades, but it has been difficult to decipher the mechanism that produces acetyl coenzyme-A in the active site of the enzyme. In our study, we report an active site model – a molecular cluster featuring two nickel atoms – which mimics the shape and size with remarkable similarity to the ACS enzyme active site.
“Excitingly, exposure of our model to carbon monoxide resulted in successful synthesis, imitating the way the ACS enzyme makes acetyl Co-A in nature.”
Scientists have been studying the ACS enzyme for decades, but it has been difficult to decipher the mechanism that produces acetyl coenzyme-A in the active site of the enzyme. In our study, we report an active site model which mimics the shape and size with remarkable similarity to the ACS enzyme active site."
Dr Daniel Wilson, Honorary Research Fellow at the Department of Science & technology Studies, University College London
Working with Dr Maxie Roessler at Imperial, the team used a technique called Electron Paramagnetic Spectroscopy to study the steps involved and believe the results will provide valuable insight for scientists studying the ACS enzyme – and other enzymes related to atmospheric carbon fixation, or carbon capture.
Dr Rebecca Musgrave said, “Our new model opens the way to better understand how this reaction works. By studying the individual reaction steps with Electron Paramagnetic Resonance spectroscopy and other techniques, we can use what we learn to inform the design of man-made catalysts for industrial use.
“This could be applied across a range of fields including new methods for capturing CO2 from the atmosphere, and using it as a feedstock to produce carbon-based chemicals such as biofuels for cars or pharmaceuticals.”
The researchers also hope that those working in enzyme spectroscopy – the field of enzyme study – will be able to take their new model and adapt it for use in their own studies.
Dr Musgrave said, “Enzymes in nature carry out these incredible transformations so quickly and efficiently, in a way that is very difficult to reproduce in the lab. Our new model brings us one step closer to understanding how these biological systems do it so well, so we can design catalysts at an industrial scale to replicate the transformative ability of nature, to tackle key societal issues such as climate change.”

