“This is the largest paediatric trial using conventional immuno-modulatory treatments in severe atopic dermatitis and was conducted across 13 centres in the UK and Ireland and is likely to change our treatment paradigm around this condition, not just for patients in the UK but also internationally.”
Professor Carsten Flohr, Chair in Dermatology and Population Health Sciences at King’s College London, and consultant dermatologist at St John’s Institute of Dermatology, Guy’s and St Thomas’ NHS Foundation Trust
19 September 2023
Clinical trial recommends methotrexate for children with severe atopic dermatitis
Findings from a clinical trial has recommended methotrexate for children with severe atopic dermatitis.
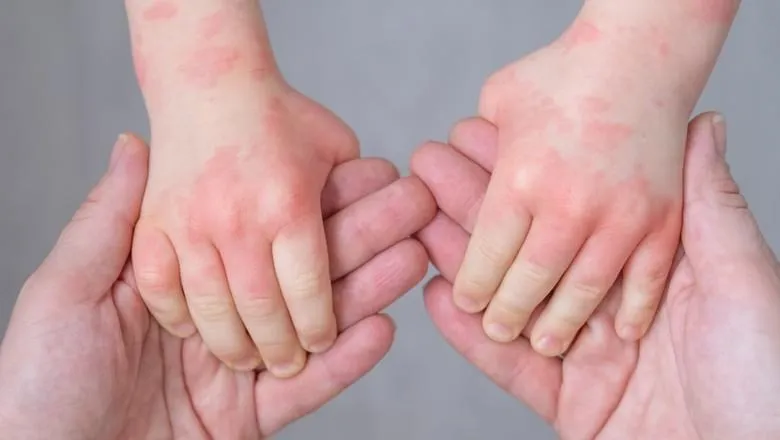
A study led by King’s College London, compared the safety and efficacy of ciclosporin with methotrexate in children and young people with this debilitating skin condition. They also examined whether the severity of the disease changed or returned after treatment ended.
For children and young people with atopic dermatitis, the most common skin condition in children, the main first line conventional systemic treatments are Methotrexate and Ciclosporin, two immuno-modulatory drugs.
However, until now there has been no adequately powered randomised clinical trial evidence in relation to their safety and treatment success for paediatric patients with this condition, and with new therapies being introduced at a high cost, establishing a gold standard for treatment with the conventional systemic therapies like methotrexate and ciclosporin is needed.
The trial assessed 103 children with severe atopic dermatitis age 2-16 years across 13 centres in the UK and Ireland. The patients were given oral doses of methotrexate or ciclosporin and assessed over nine months of treatment and six months after the therapy ended.
The study found that ciclosporin works faster and reduces disease severity more at 12 weeks but was more expensive, whereas methotrexate was significantly cheaper and led to better objective disease control after 12 weeks and off therapy, with fewer participant-reported flares of atopic dermatitis after treatment had stopped. There were also no concerning safety signals.
Based on the TREAT trial findings, methotrexate is a useful and safe treatment in paediatric patients with severe atopic dermatitis and a good alternative to ciclosporin, especially in settings where health care resources are limited.
The paper, Efficacy and safety of ciclosporin versus methotrexate in the treatment of severe atopic dermatitis in children and young people (TREAT): a multicentre, parallel group, assessor-blinded clinical trial, is published in the British Journal of Dermatology.

