This publication is really a testimony to the persistence of Sasha, a PhD student in the lab, to make the most out of the COVID restrictions, and learn how to process cryo-EM data. Her persistence turned a set of images – that we had mostly forgotten about – into a critical contribution to our understanding of photosynthesis, and our capacity to exploit this for biotechnological applications.
Dr Julien Bergeron, Senior Lecturer
05 April 2023
Cell imaging could provide next step for developing synthetic photosynthesis
An unprecedented imaging analysis of cyanobacteria provided new insights into its structure and organisation that could help synthetically replicate photosynthesis. This could have a range of biotechnological applications including in crop engineering and bioenergy.
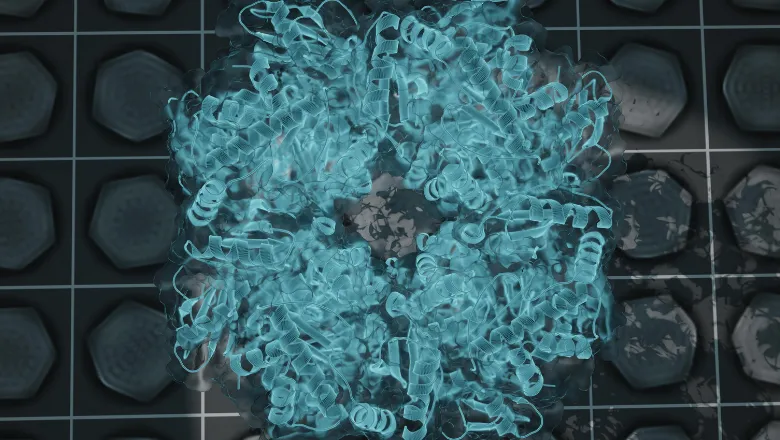
Cyanobacteria are a large group of unicellular organisms that are similar to bacteria but have the ability to perform photosynthesis – using carbon and sunlight to create oxygen and the sugars needed to keep the organism alive. This is achieved with a structure contained in its cell called carboxysomes that collect and organise all the molecules needed to kickstart the process of photosynthesis.
But the internal structure and organisation of carboxysomes are poorly understood. As an incredibly small structure, the ability to visualise and understand this structure has been impossible until recent technological advances in microscopes.
Published in Structure, the new study by King's PhD student Sasha Evans of the Bergeron Lab describes the analysis of carboxysome structure using electron microscopes. Thousands of carboxysomes images from the microscope were initially collected at the national EM facility, eBIC in Oxford, as part of a collaboration with the University of Liverpool. The data were collated and analysed by Sasha Evans during the pandemic, as an alternative project to her lab-based work that was paused due to lockdown restrictions.
The results were able to provide an unprecedented level of detail into the inner structure of the carboxysome that allowed the authors to propose models describing how enzymes are arranged and the structure of its shell. Alongside highlighting new structural features, it provided new clues as to how carboxysomes are formed within cells.
Having this knowledge is key for developing synthetic carboxysome structures that could replicate the photosynthetic process. Developing and repurposing carboxysome on an industrial scale is a subject of significant interest in biotechnology with a range of applications in crop engineering, bioenergy production, metabolic enhancement, and general therapeutics.
Professor Julien Bergeron is now hoping to use more advanced cameras that can collect millions of images per sample rather than the tens of thousands that the microscopes used for this paper are capable of. Such equipment would provide an even greater level of structural detail that could help researchers understand other aspects of carboxysomes like its dynamic properties (its reactions within constantly changing environments), or variations in sizes.

