We worked on building relationships with the largest medical schools in the country, hired a team of qualified technicians to run daily activities, and collected all requirements from the School's academics, clinical collaborators and industry partners to deliver a unique facility spearheading research, training and innovation in surgery. I am proud of the team and this achievement and look forward to seeing the impact it will have in accelerating the translation of novel medical technology into the clinic.
Dr Valentina Vitiello, Head of Clinical Translation and Governance at the London Institute for Healthcare Engineering
04 July 2024
BMEIS attains HTA license for Surgical & Interventional Engineering facilities
The Surgical and Interventional Engineering (SIE) facilities within the School of Biomedical Engineering & Imaging Sciences have attained an HTA Anatomy license to supplement their pre-clinical research.
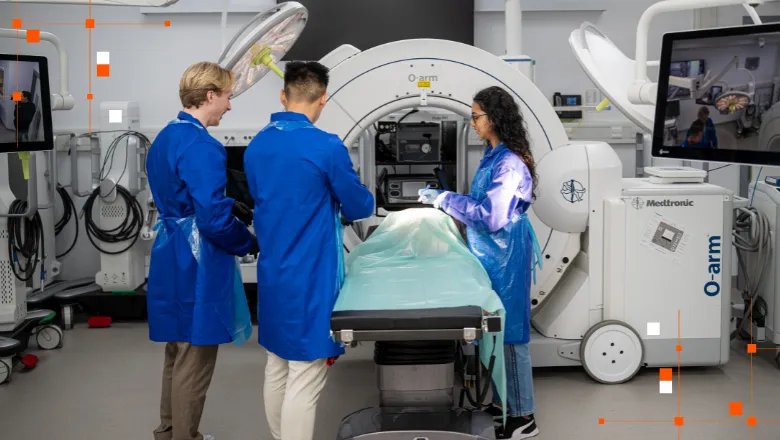
A Human Tissue Authority (HTA) license is granted under the UK's Human Tissue Act 2004. It allows for the storage and use of human tissue from the deceased for purposes including medical research, education and training.
The license obtained by the SIE is similar to those commonly in place in medical schools, where anatomical examinations are performed on human tissue as part of education and training related to human health. The procurement of human tissue for such facilities is managed by the National Repository Centre in Nottingham, which also supports members of the public who wish to donate their bodies to medical science and training.
Dr Valentina Vitiello, who is the designated individual holding the HTA license for the SIE, spoke about her experience of working towards attaining the license. "I have spent the past few years working towards attaining the HTA Anatomy license - as the prospective Designated Individual, it was my responsibility to liaise with the Human Tissue Authority, as well as the King's HTA Governance Group and relevant Health & Safety Officers, as well as the Guys and St Thomas' NHS Trust to ensure the facility and local procedures would fulfil all regulatory standards and College policies."
The license is a significant attainment for the SIE as it will allow for the development of new and innovative surgical and interventional tools to benefit future patients. One such prospective intervention comes from Dr Lukas Lindenroth, Lecturer in Surgical Robotics at the School. Dr Lindenroth is developing a new soft robotic needle guide for injections through the ear drum to treat hearing loss. The robot uses six expanding rubber balloons to stabilize itself in the ear canal and move relative to it. "We hope that with our solution, such in-the-ear injections, which are pivotal to several new hearing loss therapies, can become more precise, safer and more accessible to patients." says Dr Lindenroth.
Studies on human tissue are a crucial step to demonstrate the safety of novel devices and to trial them in one of the most realistic settings possible. Unfortunately, such studies are typically imbued with logistical challenges and high costs. Being able to conduct these studies on-site with our highly specialised equipment, as well as our experienced technical and clinical staff is a game-changer and will enable a much tighter integration in our device development and verification process which will, in return, allow us to bring new technologies to our patients faster.
Dr Lukas Lindenroth, Lecturer in Surgical Robotics
This makes the SIE one of only two facilities in London with a co-located mock operating theatre and human tissue repository, allowing it to close the operational gap between device development and testing. The facility is open to researchers across and beyond King's.
We are pleased to have obtained an HTA license for our SIE facilities. This is an integral step in our mission to translate innovations from bench to bedside to boardroom at pace. The research carried out here will allow us to safely validate technology in the most realistic pre-clinical environment, provide training to clinicians on how to use new devices, and strengthen collaboration with our existing and prospective industry partners.
Professor Sebastien Ourselin FREng FMedSci, Head of the School of Biomedical Engineering & Imaging Sciences



