As described in the Motor Neurons section, a motor unit comprises the lower motor neuron, its terminal branches and the muscle fibres it connects to. MUNE is a technique that assesses the number of viable motor units activating a muscle at a particular point in time.
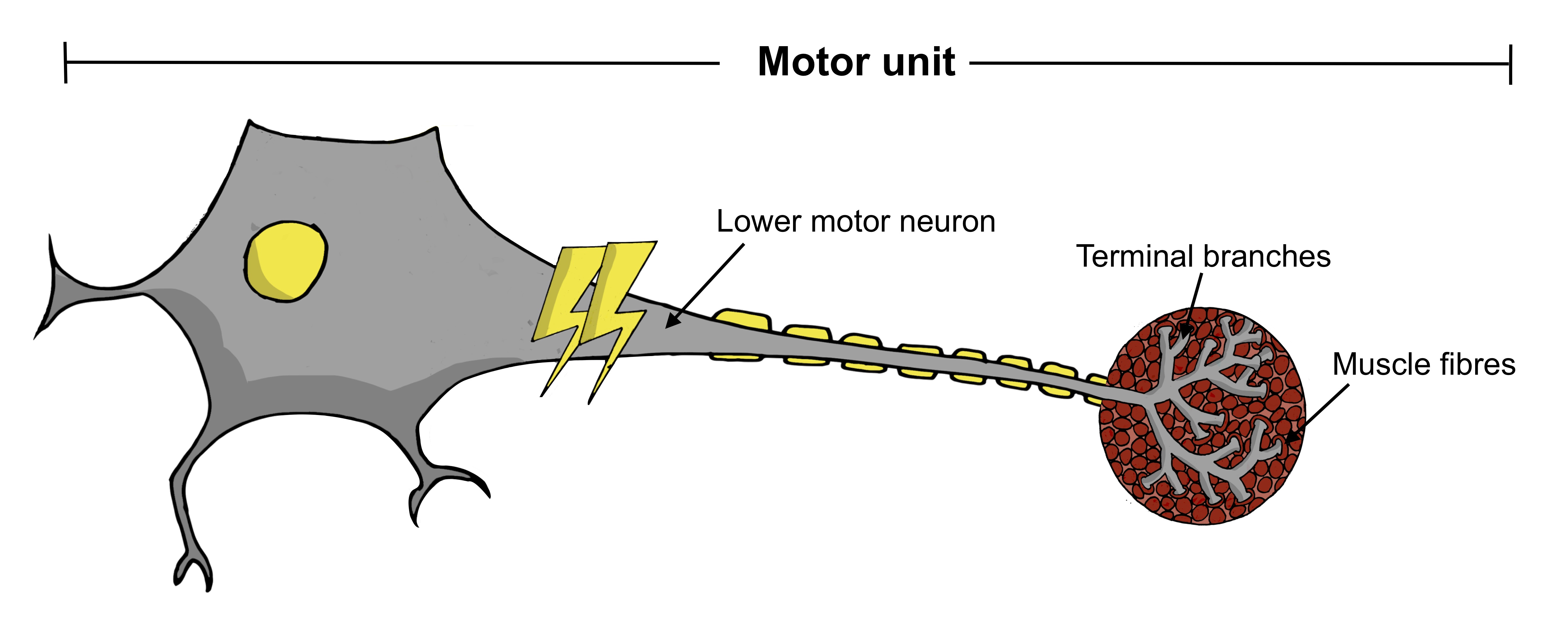
The MUNE techniques have evolved significantly over the last 50 years, and the modern methods are non-invasive and generally well-tolerated by patients. They are therefore suitable for repeated assessment of patients over a longer period of time. One of the more recent techniques, the motor unit number index (MUNIX), is noteworthy for its rapidity, whereby each muscle can be assessed in under five minutes.
How can MUNE be applied to motor neuron disease?
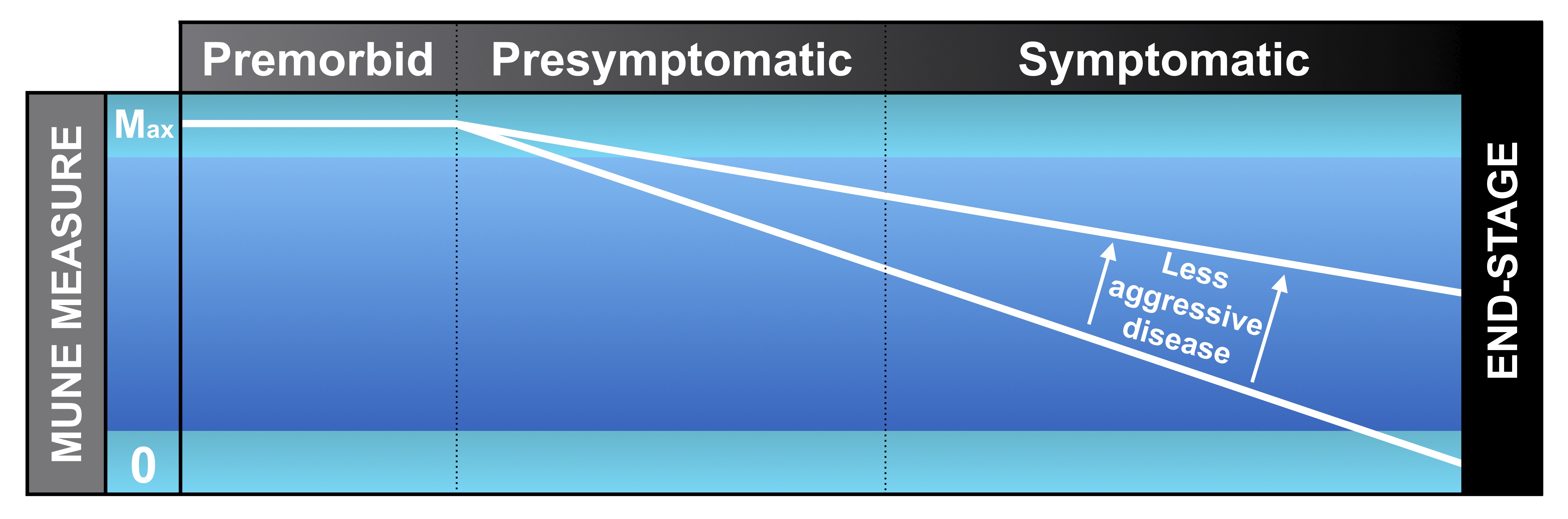
Motor neuron disease is characterised by the progressive loss of motor units, consequently resulting in muscle weakness. It is therefore of great interest to estimate the number of surviving functional motor units in order to study disease progression.
Natural history studies have demonstrated the utility of MUNE measures, particularly MUNIX, to reliably track patients both before and after the onset of symptoms in individual muscles. Given the ability to calculate an average rate of decline in MUNIX, it is possible to test whether a new therapy slows or halts progression. It is anticipated that this quantitative approach should be much more powerful than current outcome measures in clinical drug trials.
What are we adding to MUNE research?
We have an ongoing project at King’s, funded by the Motor Neurone Disease Association, that will explore a new MUNE method, which no longer requires electrical stimulation. This so-called ‘stimulation-free’ MUNE should improve tolerance amongst patients, whilst allowing greater accessibility to the technique.
In parallel with our efforts to introduce a home muscle recording device, the new stimulation-free MUNE will uniquely be collected directly in patients’ homes in the absence of an expert researcher. This would avoid burdensome travel for patients to hospital-based research centres and provide the means to assess patients much more frequently in clinical drug trials (e.g. weekly). All these attributes could provide a meaningful breakthrough in the search for novel therapies in MND.
How can MUNE be calculated without electrical stimulation?
At the moment, we are not sure what the precise answer to this question is. However, we have good reason to believe it can be achieved via a method known as motor unit decomposition. Essentially, this involves a complex computer algorithm that scans recordings from high-density surface EMG, looking for individual motor unit signatures. This relies on the fact that every time a motor unit is active, it produces its own characteristic amplitude ‘fingerprint’ on the EMG trace. By identifying these fingerprints, we can learn about the firing of individual neurons, all simply by placing a sophisticated sticker on the skin!
We tested this method on data collected from MND patients, demonstrating the feasibility of this approach. We were able to show that some motor units adapted from ‘slow-twitch’ to ‘fast-twitch’ as disease progressed, something that is valuable to know as we move on to develop a stimulation-free MUNE. See our Publications page for a link to the full research paper.
What is home EMG monitoring?
EMG (electromyography) is the process of recording electrical muscle activity. Traditionally, this is performed using a fine needle placed into the muscle for a few seconds. More recently, non-invasive surface EMG has gained appeal, as it is more comfortable for patients and can record for up to 30 minutes.
Home EMG is when surface EMG is applied in the patient’s home. Useful parameters, such as fasciculation frequency, can be calculated. Multiple sessions can then be conducted at relatively short intervals (e.g. weekly) to build up an insightful profile of the disease process.
At least, this is the aim! The implementation of such a platform is fraught with challenges and remains a key focus of our research at King’s.
When will home EMG monitoring become available?
We are currently in the process of validating our new portable EMG device (named Panacea), which was designed in collaboration with our bioengineering colleagues at Imperial College London (Professor Emmanual Drakakis and Dr Georgios Zafeiropoulos). During a recent feasibility study, this device was used for the first time on 12 patients with MND in both hospital and home environments. We hope to report on these results in 2022.
At the same time, we are setting up a bigger home monitoring study as part of a Cross-Collaboration Grant with Professor David Sharp (Imperial College London), funded by the UK Dementia Research Institute. This will incorporate the Panacea EMG device into an established home monitoring platform originally designed for patients with dementia. This platform includes door sensors, sleep mats and wearables to compile a comprehensive picture of patient activity. By collecting such a rich dataset over the course of a year, we hope to identify novel patterns of disease progression that could undergo subsequent validation for clinical drug trials.
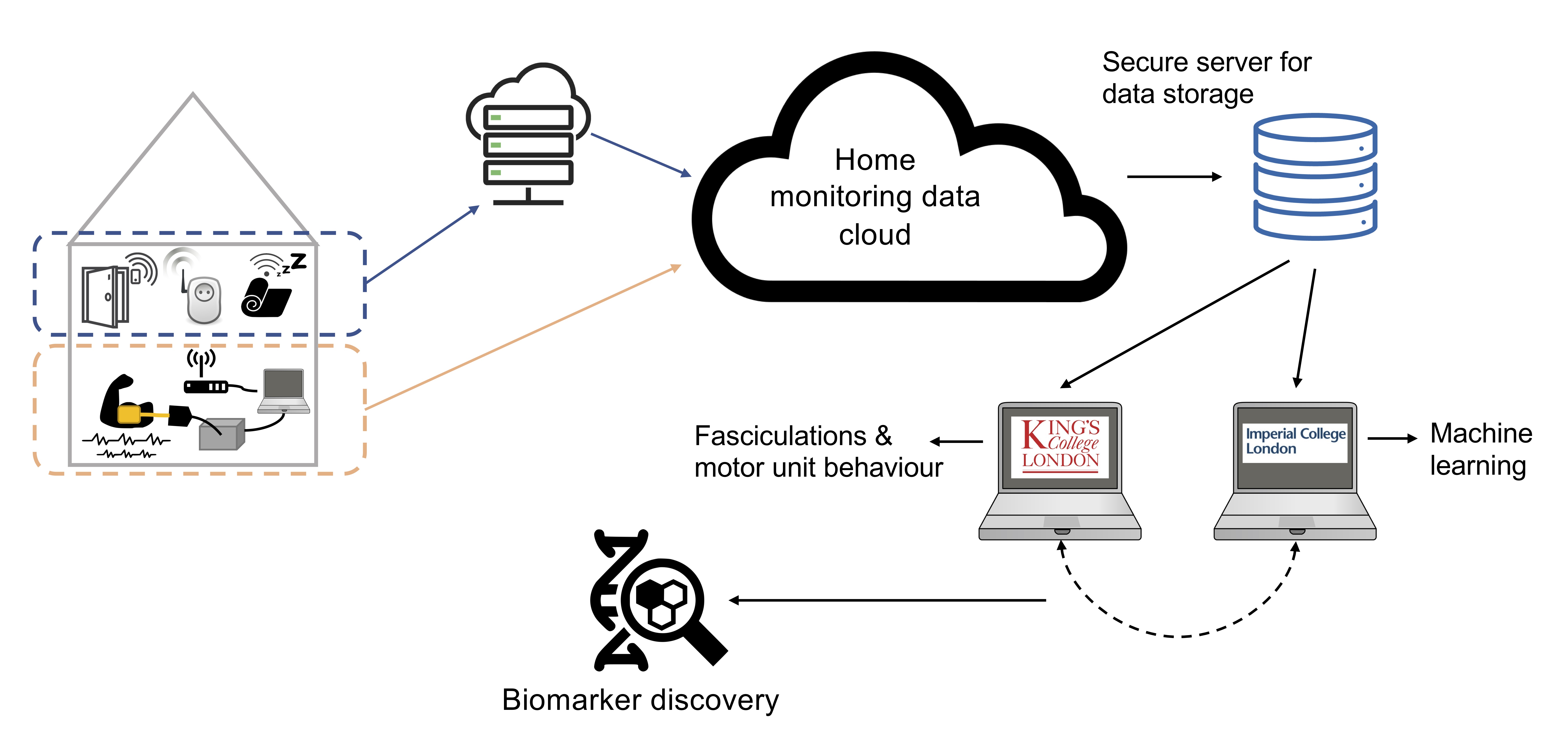
This larger project will run until 2024, at which time we hope to have a clearer picture of the utility and practicalities of home EMG monitoring.
The Panacea EMG device
To learn more about how the Panacea EMG device is setup in either the hospital or home, please watch this short video.
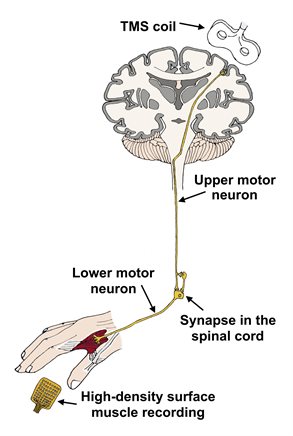
What is transcranial magnetic stimulation (TMS)?
During TMS, motor neurons in the brain are activated by an external magnetic coil placed over the scalp. This has been safely tested in humans for many years, showing recognisable changes in patients with motor neuron disease. These changes indicate alterations in the degree of excitability of motor neurons in the brain. Typically, there are signs of increased excitability in the early stages of disease.
While our research into fasciculations tells us principally about lower motor neuron dysfunction, TMS highlights impairment of the upper motor neuron (please visit our Motor Neurons page to learn more about these two types of neurons). Damage to both types of neuron is characteristic of motor neuron disease, so it is important to build up a variety of techniques in order to understand the complete disease process.
What is novel about our TMS research?
We combine TMS with high-density surface muscle recordings instead of the single-channel muscle recordings that are traditionally used. We anticipate that this will improve the accuracy of the technique, while teaching us more about motor connections at the spinal level.
We are also employing TMS with brain recordings (EEG, electroencephalography). Although this method has been widely employed to study the effects of anti-epileptic medications, it has not been studied in patients with motor neuron disease. We suspect this approach may inform changes in brain excitability that occur as a result of the disease.
What does ultrasound add?
Ultrasound is widely used in medicine to provide quick, safe images of various parts of the body. Well known as the modality of choice for visualising a foetus in the womb, ultrasound has grown in popularity amongst doctors and researchers, serving as a useful tool to assess nerves and muscles too.
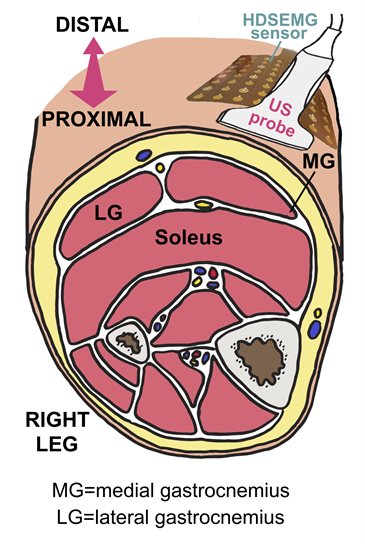
In the context of MND research, ultrasound can detect the mechanical component of fasciculations. We have teamed up with Dr Emma Hodson-Tole (Manchester Metropolitan University), who has an expertise in this area.
How have we applied ultrasound to our research?
We completed a study of 12 MND patients and 13 healthy participants, combining ultrasound and high-density surface EMG simultaneously. This allows us to investigate both the electrical and mechanical properties of fasciculations at the same time, informing us about how neuromuscular function breaks down in MND in a new way.
We are in the process of finalising this analysis and aim to submit this work for publication soon.